In response to customer demands to deliver projects more quickly and cheaply, design firms increasingly are looking at modular design and construction to streamline the construction process. Many clients put a premium on tight schedules where a product’s speed to market is critical. Few customers place more emphasis on this issue than the pharmaceutical industry.
As a result, the pharmaceutical industry has long embraced modular construction. In response, IPS-Integrated Project Services LLC (No. 124), one of the nation’s largest pharmaceutical plant engineers, has partnered with G-Con Manufacturing Inc., a College Station, Texas-based manufacturer of prefabricated cleanroom systems, to create iCON. The design and construction process rapidly deploys modular and mobile pharmaceutical research and production facilities.
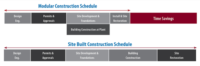
Through iCON, IPS works with G-Con to design and specify the facility, while G-Con fabricates the cleanrooms locally for shipment to the assembly site. Further, IPS acts as construction manager and hires general contractors to assemble the building quickly (see above). “We might hire someone like Butler to fabricate and assemble a manufactured building quickly to house the production facility,” says Tom Piombino, vice president for IPS.
One of the great advantages is that G-Con can fabricate the cleanroom pods in low-cost Texas and simply ship them to high cost locations like San Francisco, Piombino says. Further, the fabrication process can start even before final permitting of the site has been completed, rather than wait for site access. This can cut the total construction time down to 12-18 months, from as much as 36 months. “Where getting the product to market is critical, that makes a big difference,” he says.
“We have competitors like Jacobs that are developing their own modular approaches to pharmaceutical plant construction. However, iCON is the only process so far that is designed to be fully mobile and reusable,” Piombino says.
Piombino says that the process already has drawn customers from smaller pharmaceutical companies. For example, IPS and G-Con are currently constructing a production facility in the Northeast for a small pharmaceutical company to produce an oncology drug for clinical trials to treat multiple myeloma. But larger companies already have shown a great deal of interest. “It has only been six months since we launched iCON, but the market is very excited,” Piombino says.
Related Article: ENR 2018 Top 500 Design Firms: Worry Despite Strong Markets




Post a comment to this article
Report Abusive Comment